
AACR Cancer Progress Report 2023 Chronicles Latest Wave of Advances
Patient stories show real-world results of cancer research breakthroughs.
Courtney Addison is eternally grateful for the CAR T-cell therapy that sent her son Cayden’s leukemia into remission.
But her heart aches for the long journey her little boy endured after receiving a diagnosis of Philadelphia chromosome-positive acute lymphoblastic leukemia when he was only 3 years old. His treatment began with chemotherapy, which failed to produce lasting results and left Cayden suffering from punishing side effects, including a life-threatening case of sepsis.

Cayden experienced a brief remission, but his cancer relapsed in February 2023. “He only had 9 months of normalcy,” his mother said at a September 2023 briefing for the AACR Cancer Progress Report 2023. Because his type of leukemia is characterized by a high risk of relapse, Cayden’s doctors recommended that he try CAR T-cell therapy, in which a patient’s T cells are extracted, engineered to target cancer cells, expanded, and infused back into the patient.
The therapy was successful, producing a complete remission with minimal side effects. Cayden turned 7 in September 2023. He’s back in school and loves playing with his older brother, Christian, and his friends. He may still need further treatment, but for the moment, his mother joined with the American Association for Cancer Research® (AACR) to celebrate his progress and call for continued robust federal funding for medical research.
“We wouldn’t be here if it wasn’t for the research,” Addison said. Going forward, she hopes researchers will be able to design cancer treatments that are less toxic to pediatric patients.
Cayden’s story is featured in the AACR Cancer Progress Report 2023, which chronicles how basic, translational, and clinical cancer research and cancer-related population sciences—primarily supported by federal investments in the National Institutes of Health (NIH) and the National Cancer Institute (NCI)—remain vitally important to improving health and saving lives. Along with Cayden’s experience, the report features personal stories from several other patients who have benefited from innovative, recently approved anticancer therapeutics, highlighting the real-world impact of cancer research.

The report was released at a September 13, 2023, briefing held in the National Press Club in Washington, D.C., with nearly 50 people attending and more than 300 livestreaming the event. Nearly two dozen media members covered the release.
“The advances in cancer research, particularly in the last two decades, have been breathtaking. We are in an era of unparalleled opportunity to make even more breakthroughs for patients,” Philip D. Greenberg, MD, AACR president, said at the briefing. “For the cancer research community to achieve these breakthroughs, however, our representatives in Congress must continue to prioritize funding for biomedical research, from basic research to clinical trials. Through the AACR Cancer Progress Report 2023, we are sharing with the public and policymakers the progress that has been made, so they understand how crucial it is that we maintain this momentum through continued support of NIH and NCI.”
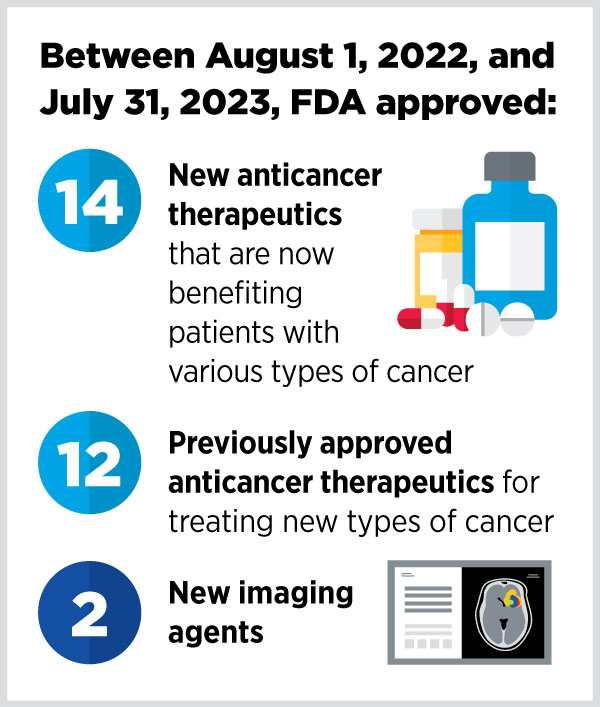 Each year, the report highlights the cancer therapeutics that the U.S. Food and Drug Administration (FDA) approved in the previous year. From August 1, 2022, to July 31, 2023, the FDA approved 14 new anticancer therapeutics and two new imaging agents, and it expanded the use of 12 previously approved anticancer therapeutics to treat additional cancer types.
Each year, the report highlights the cancer therapeutics that the U.S. Food and Drug Administration (FDA) approved in the previous year. From August 1, 2022, to July 31, 2023, the FDA approved 14 new anticancer therapeutics and two new imaging agents, and it expanded the use of 12 previously approved anticancer therapeutics to treat additional cancer types.
Among the new approvals was an immunotherapy drug that effectively treated Lesa Kirkland’s bladder cancer. “Sufficient funding from Congress will allow the doctors and scientists and chemists to think outside the box, to develop the next round of treatments without worrying about where their next round of funding is coming from,” Kirkland said at the briefing.





